|
Case Report: Deep Transcranial Magnetic Stimulation (dTMS) and Improvement of Kidney Functions in a 72 year-old female CKD patient Jacek Maitan (jmaitan@mvptms.com) 2016 |
|
|||||||
|
ABSTRACT: The patient is a nonAfrican female 72 yrs, 80 kG. Major increase of eGFR from 40 to 51 [mL/min/1.73] and reduction of creatinine serum from 1.35 to 1.09 [mg/dL] was detected after 24 sessions of dTMS (Brainsway) of Major Depressive Disorder (MDD); lab tests were performed within 4 months. This case may perhaps be a potential indicator of functional improvement that can be gained by integrating psychosomatic view into the clinical practice of nephrology. |
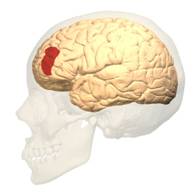
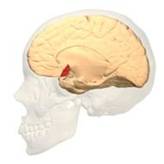
BA 46 |
|
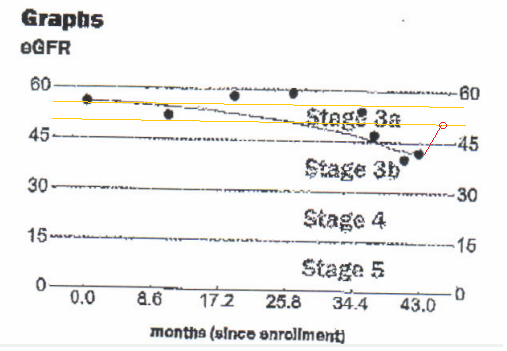
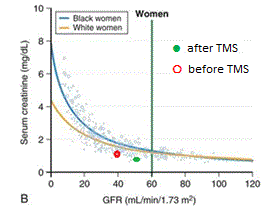
Patient history Fig. 2a and 2b indicates a steady decline of renal functions. An unexpected change in this trend as measured by eGFR and creatinine [4] that occurred after the TMS. Both creatinine and eGFR improved and their values moved toward the normal range. These changes that coincide with TMS treatments are perhaps worth to notice and should be followed by a study based on a larger cohort.
|
|||||
|
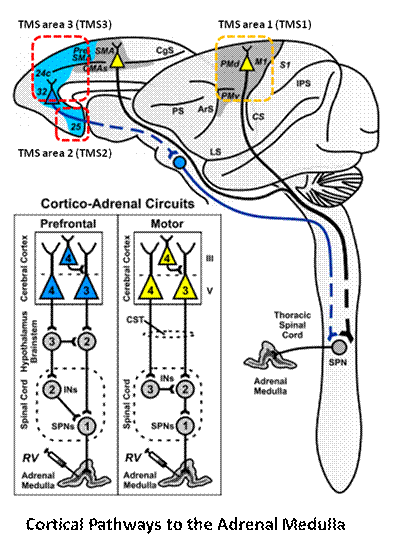
THEORY: Cortico-adrenal circuits are a multisynaptic circuits that connect neurons in the motor and prefrontal areas of the cerebral cortex to the adrenal medulla. When rabies virus is injected into the adrenal medulla, it is transported transneuronally in the retrograde direction through these circuits. Depending on the survival time, the virus will infect first-order (1), second-order (2), third-order (3), or fourth-order (4) neurons.
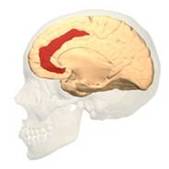
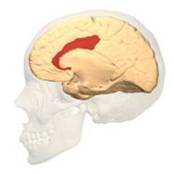
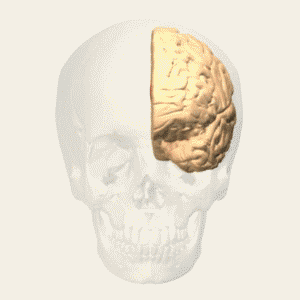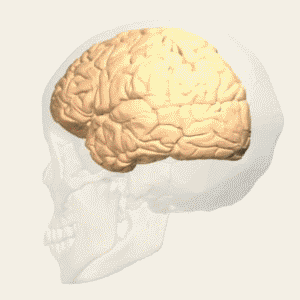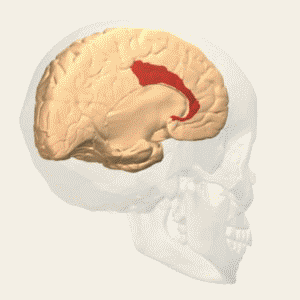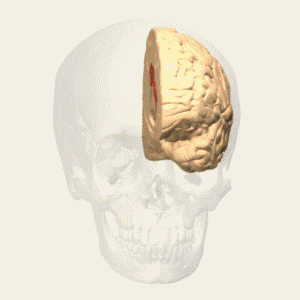
Fig. 3 Two Cortical Pathways to Adrenal Medulla open new TMS treatment opportunities [2, 3] ArS, arcuate sulcus; CgS, cingulate sulcus; CMAs, cingulate motor areas; CS, central sulcus; CST, corticospinal tract; INs, interneurons; IPS, intraparietal sulcus; LS, lateral sulcus; M1, primary motor cortex; MN, motoneurons; PMd and PMv, dorsal and ventral premotor areas; PS, principal sulcus; S1, primary somatosensory cortex; SMA, supplementary motor area; SPNs, sympathetic preganglionic neurons.[from [2]]
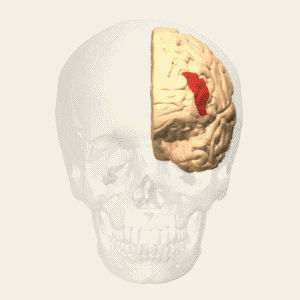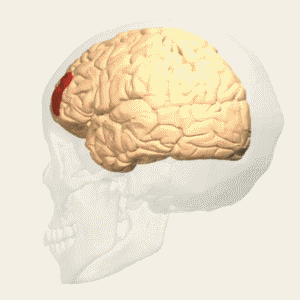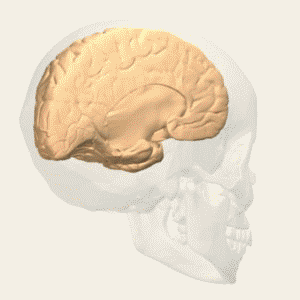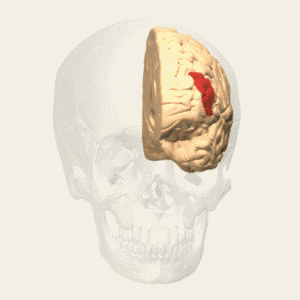
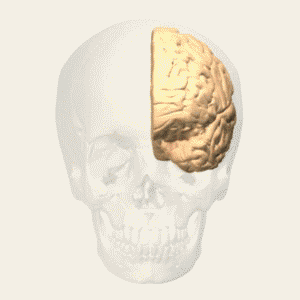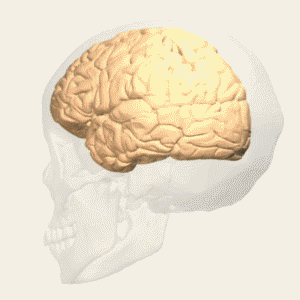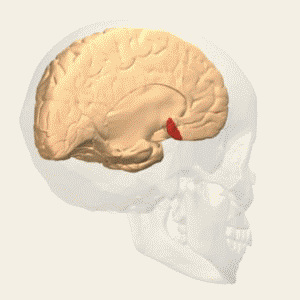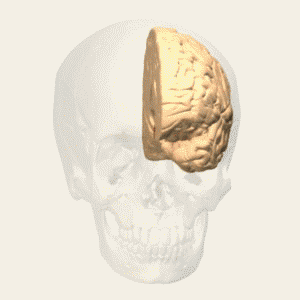
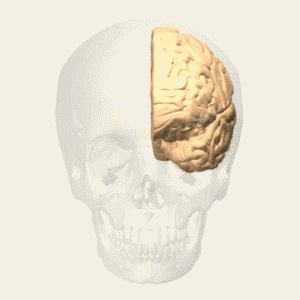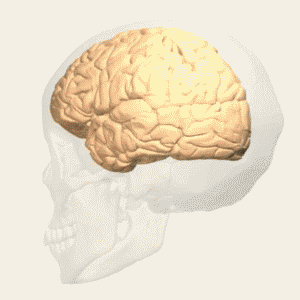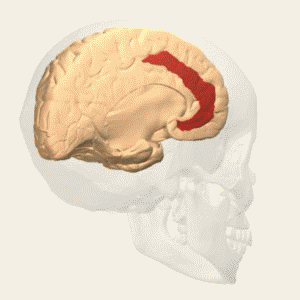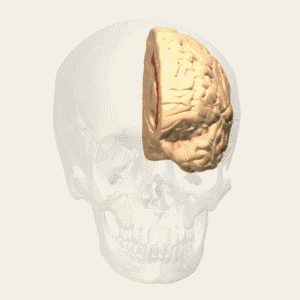
Gray shading, cortical motor areas; blue shading, medial prefrontal areas. In the case described in this note, we have treated MDD, improvement of CKD was just a side effect. Our treatment used a deep penetration H-coil with broad action area. This may have resulted in an additional side effects where a wide area of prefrontal cortex is being affected in a fashion which may need to be determined. TMS area 1 (TMS1) corresponds to the area used in [3]. TMS areas TMS2 and TMS3 are the areas involved in stimulation of BA25 using Brainsway's H-coil. Stimulation using TMS BA 46 also affects BA 25 [4] and this leads to a hypothesis that this link explains the effect of the treatment, this area is labeled TMS2. |
||||||||
|
BA 25 |
|
|
||||||
|
BA 32 |
|
|||||||
|
BA 24 |
(3D brain data is from Anatomography.) |
DISCUSSION: TMS stimulation of motor cortex and its impact on kidneys has been explored on the premise that there is a afferent link with renal control. A figure-of-eight coil set up at 90% of motor evoked potential (MEP) has been applied to stimulate motor cortex of diabetic patients (area labeled TMS1) and as a result proteinuria and albuminuria were improved after 5 treatments [3]. The case described here indicates that perhaps stimulation of prefrontal part of cortico-adrenal circuits can be achieved in an FDA approved protocol for MDD treatment using TMS. This also confirms the clinical value of the model described in [2, 4]. If the hypotesis presented here will be confirmed, then a treatment protocol combining the approach described in [3] offers perhaps a novel and effective approach to some cases of CKD. The collocation of neural mechanisms controlling autonomous and emotional state may explain the impact of the treatment on both function of kidneys and depression. The existence of emotional problems in CKD patients in dialysis has been reported. Thus, it seems there is perhaps a need for augmenting CKD treatment to include mental component, TMS which is a non-pharmacological, non-invasive treatment of MDD may perhaps be also a right auxiliary treatment of their autonomous functions including kidneys. An open question to be answered by a lerge scale studies is whether in cases of CKD patients, MDD diagnosis is necessary to start the TMS treatment. References: 1. T. Perera et al, The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder, Brain Stimulation, MayâJune, 2016Volume 9, Issue 3, Pages 336â346, 2. Richard P. Dum et al, Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla, PNAS submission March 2016, 3. Rosaria Lupica, et al, Proteinuric effect of transcranial magnetic stimulation in healthy subjects and diabetic patients with Stage 3â4 CKD, Nephrol Dial Transplant (2013) 4. Fox, MD; at all (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry. 72 (7): 595â603. 5. Zhi-De Deng, Coil Design Considerations for Deep Transcranial Magnetic Stimulation, Clin Neurophysiol. 2014 June; 125(6): 1202:1212 This draft material is for private distribution only. For comments and interest in participating in a larger study please send an email. .Rev. 0.5 Nov.28.2016 |
|
|||||
|
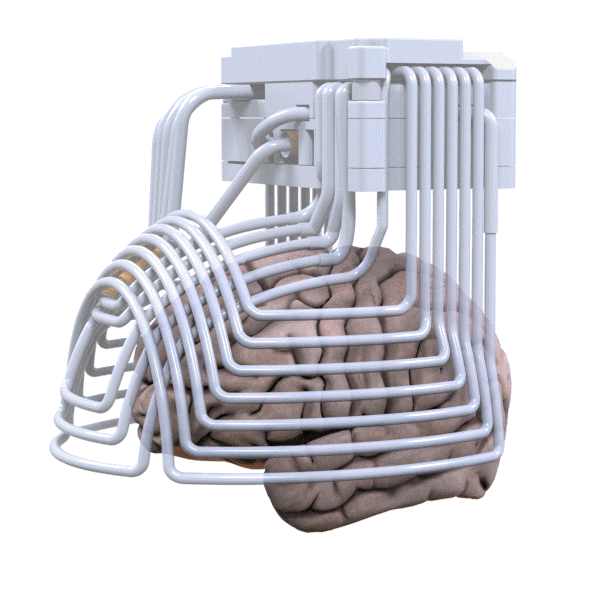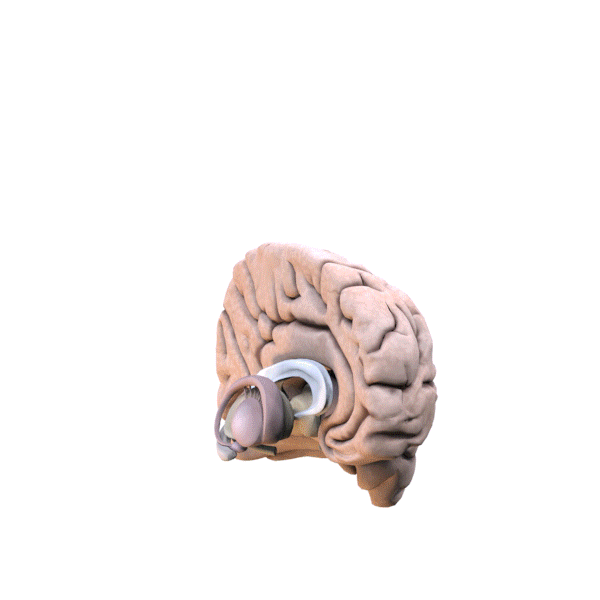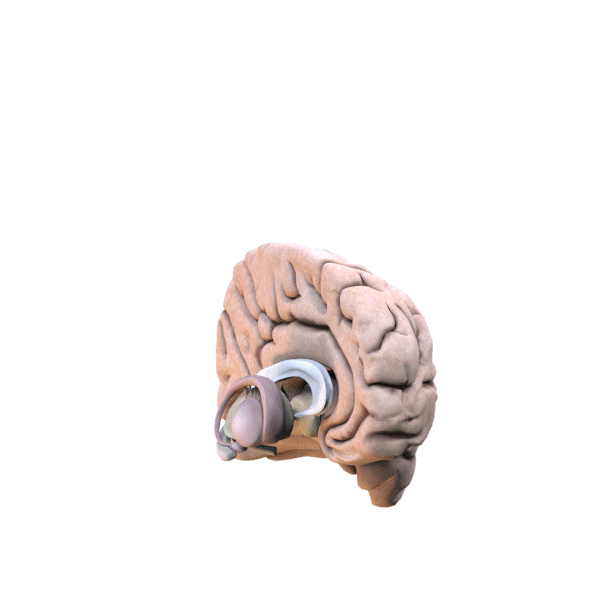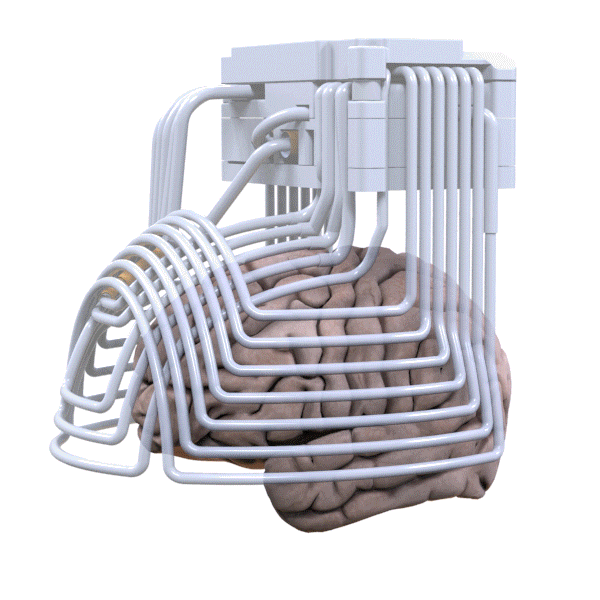
Fig. 4 Brainsway H-coil has a deep and broad field of stimulation [5] |
Fig. 5 Figure-8 coil a smaller spot and depth of stimulation [5] |
|
||||||